FluoTag®-Q anti-RFP
400,00 €
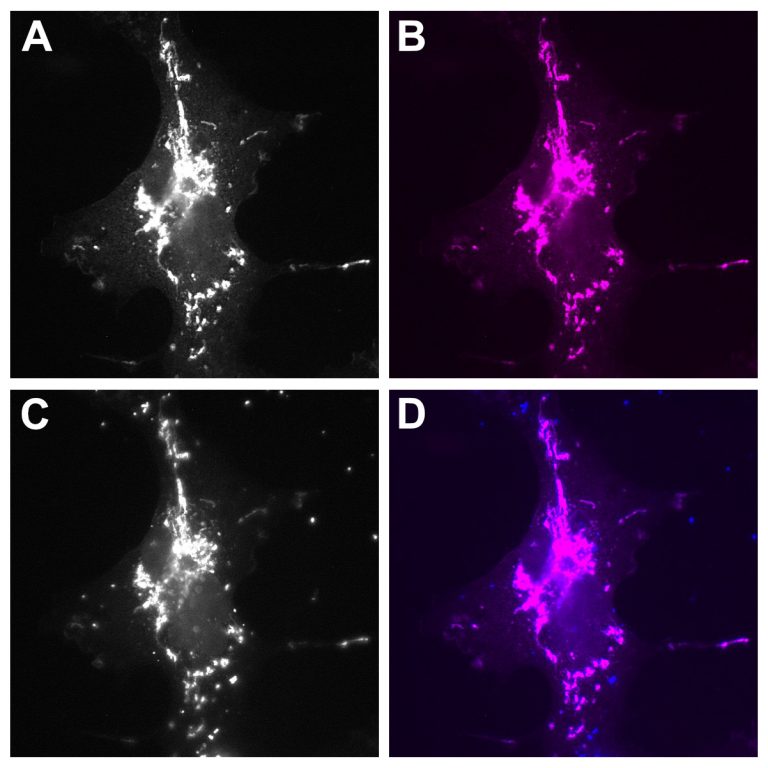
FluoTag®-Q anti-RFP is derived from an in-house developed single-domain antibody (sdAb) recognizing the most common red fluorescent proteins like mRFP, mCherry, DsRed and other DsRed derivatives with high affinity and specificity.
One of the first red-emitting fluorescent proteins (FP) described was DsRed from Discosoma sp. Only shortly after its publication in 1999 and including more than 20 mutations, a monomeric version with several enhanced characteristics was discovered, mRFP1. From this promising monomeric red-FP, dozens of monomeric variants have been described, from which mCherry represents one of the most popular derivatives. Today more than 800 entries of various fluorescent proteins can be found in an open-source database with the most currently available variants in the fluorescence protein database “fpbase”
Our nanobodies bind specifically and strongly to DsRed, RFP, and mCherry, among other family members. Look at our specificity chart in the Resource Section.
FluoTags® can be equipped with a single fluorophore for more quantitative readouts (FluoTag-Q), with two fluorophores per single-domain antibody (FluoTag-X2), and we also developed a blend of two sdAbs bindings simultaneously the target proteins and each bearing two fluorophores (FluoTag-X4). For more detailed information on the FluoTags, please check our Technology Section.
| Variations: |
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Related Products: | - | ||||||||||||||||||||||||
| Clone: | 2B12 | ||||||||||||||||||||||||
| Host: | Alpaca | ||||||||||||||||||||||||
| Produced in: | E.coli | ||||||||||||||||||||||||
| Application: | IF | ||||||||||||||||||||||||
| Dilution: | 1:1000 (corresponding to 5 nM final concentration) | ||||||||||||||||||||||||
| Capacity: | N/A | ||||||||||||||||||||||||
| Antigen: | - | ||||||||||||||||||||||||
| Targets: | mRFP | ||||||||||||||||||||||||
| Specificity: | It recognizes RFP (red fluorescent protein) and other RFP-derivatives like mOrange, dsRed1, dsRED2, tdTomato, mCherry and mScarlet-i. Probably also other derivatives not yet tested. | ||||||||||||||||||||||||
| Formulation: | A single sdAb clone was lyophilized from PBS pH 7.4 containing 2% BSA (US-Origin). Reconstitute with 200 µL of 50 % glycerol in deionized water. We recommend including 0.1 % sodium azide as a preservative if applicable. When reconstituted in 200 µl, the concentration of single-domain antibody is 5 µM | ||||||||||||||||||||||||
| kDa: | - | ||||||||||||||||||||||||
| Ext Coef: | - | ||||||||||||||||||||||||
| Shipping: | Ambient temperature | ||||||||||||||||||||||||
| Storing: | Vials containing lyophilized protein can be stored at 4 °C for 6 months. We recommend reconstituting the protein with 50 % glycerol including 0.1 % sodium azid as preservative if applicable. Minimize the number of freeze-thaw cycles by aliquoting the reconstituted protein. Long term storage at -80 °C up to 6 months. Working aliquots can be stored at -20 °C for up to 4 weeks. | ||||||||||||||||||||||||
| Protocols: |
Western Blotting is not recommended with this product, sdAbs tend to recognize native protein conformation only. Look at detailed protocols and our specificity chart in our Resource Section. |
||||||||||||||||||||||||
| References: |
|
||||||||||||||||||||||||
| Notice: | To be used in vitro/ for research only. Non-toxic, non-hazardous, non-infectious. | ||||||||||||||||||||||||
| Legal terms: | By purchasing this product you agree to our general terms and conditions. |